Doubled Pregnancy Loss Rate, Raised Foetal Abnormality Rate and Concentration of Lipid Nanoparticles in Ovaries – How Could They Call This Vaccine ‘Safe’?

Global Research | Alex Kriel
The mRNA vaccines were released globally in early 2021 with the slogan ‘safe and effective’. Unusually for a new class of medicine, they were soon recommended by public health authorities for pregnant women. By late 2021, working age women, including those who were pregnant, were being thrown out of employment for not agreeing to be injected. Those who took the mRNA vaccines did so based on trust in health authorities – the assumption being that they would not have been approved if the evidence was not absolutely clear. The role of regulatory agencies was to protect the public and, therefore, if they were approved, the drugs were safe.
Recently, a lengthy vaccine evaluation report (above) sponsored by Pfizer and submitted to the Australian regulator, the Therapeutic Goods Administration (TGA) dated January 2021 was released under a Freedom of Information request. The report contains significant new information that had been supressed by the TGA and by Pfizer itself. Much of this relates directly to the issues of safety in pregnancy and impacts on the fertility of women of child-bearing age. The whole report is important, but four key data points stand out:
- The rapid decline in antibody and T cells in monkeys following a second dose;
- Biodistribution studies (previously released in 2021 through an FOI request in Japan);
- Data on the impact of fertility outcomes for rats;
- Data on foetal abnormalities in rats.
We focus on the last three items as, for the first point, it is enough to quote the report itself: “Antibodies and T cells in monkeys declined quickly over five weeks after the second dose of (Pfizer Covid vaccine) BNT162b2 (V9), raising concerns over long term immunity.” This point indicates that the regulators should have anticipated the rapid decline in efficacy and must have known at the outset that the initial two dose course was unlikely to confer lasting immunity and would, therefore, require multiple repeat doses. This expectation of failure was recently highlighted by Dr. Anthony Fauci, former director at the U.S. NIH.
The three remaining items should be a major cause for concern with the pharmaceutical regulatory system. The first, as revealed in 2021, involved biodistribution studies of the lipid nanoparticle carrier in rats, using a luciferase enzyme to substitute for the mRNA vaccine. The study demonstrated that the vaccine will travel throughout the body after injection and is found not only at the injection site but in all organs tested, with high concentration in the ovaries, liver, adrenal glands and spleen. Authorities who assured vaccinated people in early 2021 that the vaccine stays in the arm were, as we have known for two years, not being honest.
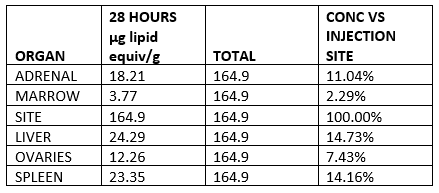
Lipid concentration per gram, recalculated as percentage of injection site.
In terms of the impact on fertility and foetal abnormalities, the report includes a study of 44 rats and describes two main metrics, the pre-implantation loss rate and the number of abnormalities per foetus (also expressed per litter). In both cases the metrics were significantly higher for vaccinated rats than for unvaccinated rats.
Roughly speaking, the pre-implantation loss ratio compares the estimated number of fertilised ova and the ova implanted in the uterus. The table below is taken from the report itself and clearly shows the loss rate for vaccinated (BNT162b2) is more than double the unvaccinated control group.
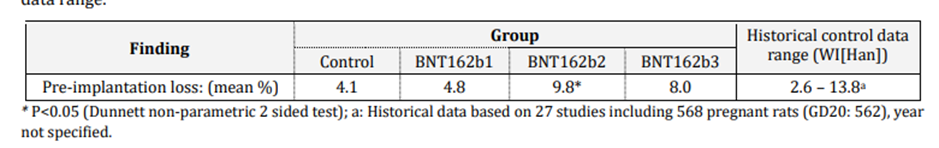
In a case control study, a doubling of pregnancy loss in the intervention group would represent a serious safety signal. Rather than take this seriously, the authors of the report then compared the outcomes to historical data on other rat populations – 27 studies of 568 rats – and ignored the outcome because other populations had recorded higher overall losses. This range is shown in the right hand column as 2.6% to 13.8%. This analysis is alarming as remaining below the highest previously recorded pregnancy loss levels in populations elsewhere is not a safe outcome when the intervention is also associated with double the harm of the control group.
A similar pattern is observed for foetal malformations with higher abnormality rate in each of the 12 categories studied. Of the 11 categories where Pfizer confirmed the data are correct, there are only two total abnormalities in the control group, versus 28 with the mRNA vaccine (BNT162b2). In the category which Pfizer labelled as unreliable (supernumerary lumbar ribs), there were three abnormalities in the control group and 12 in the vaccinated group.
As with the increased pregnancy losses, Pfizer simply ignored the trend and compared the results with historical data from other rat populations. This is very significant as it is seen across every malformation category. The case control nature of the study design is again ignored, in order to apparently hide the negative outcomes demonstrated.
These data indicate that there is no basis for saying the vaccine is safe in pregnancy. Concentration of lipid nanoparticles in ovaries, a doubled pregnancy loss rate and raised foetal abnormality rate across all measured categories indicates that designating a safe-in-pregnancy label (B1 category in Australia) was contrary to available evidence. The data imply that not only was the Government’s ‘safe and effective’ sloganeering not accurate, it was totally misleading with respect to the safety data available.
Despite the negative nature of these outcomes, the classification of this medicine as a ‘vaccine’ appears to have precluded further animal trials. Historically, new medicines, especially in classes never used in humans before, would require a very rigorous assessment. Vaccines, however, have a lower burden of proof requirement than ordinary medicines. By classifying mRNA injections as ‘vaccines’, this ensured regulatory approval with significantly less stringent safety requirements, as the TGA itself notes. In fact, these mRNA gene therapy products (to use the proper term) function more like medicines than vaccines in that they modify the internal functioning of cells, rather than stimulating an immune response to presence of an antigen. Labelling these gene therapy products as ‘vaccines’ means that, as far as we are aware, even today no genotoxicity or carcinogenicity studies have been carried out.
This report, which was only released after a FOI request, is extremely disturbing as it shows that authorities knew of major risks with mRNA COVID-19 vaccination while simultaneously assuring populations that it was safe. The fact that mainstream media have (as far as we are aware) completely ignored the newly released data should reinforce the need for caution when listening to the advice of public health messaging regarding COVID-19 vaccination.
Firstly, it is clear that regulators, drug companies and the Government would have known that vaccine induced immunity tails off very rapidly, with this being subsequently observed in real world data with efficacy against infection falling to zero. Accordingly, the single point in time figures of 95% and 62% efficacy against symptomatic [fake test looking for nothing] PCR-positive infection quoted for Pfizer and AstraZeneca respectively meant almost nothing since a rapid decline was to be expected. Similarly, the concept of a two dose course was inaccurate as endless boosters would likely have been required given the rapid decline in antibodies and T-cells observed in the monkeys.
Most importantly, the data do not in any way support the ‘safe’ conclusion with respect to pregnancy; ‘dangerous’ would be much more accurate. The assurances of safety were, therefore, completely misleading given the data disclosures in the recent freedom of information release. Regulatory authorities knew that animal studies showed major red flags regarding both pregnancy loss and foetal abnormalities, consistent with the systemic distribution of the mRNA they had been hiding from the public. Even in March 2023, it is impossible to give these assurances, given the fact that important studies have not, to the best of our knowledge, been done. Pfizer elected not to follow up the vast majority of pregnancies in the original human trials, despite high miscarriage rates in the minority they did follow. Given all of the problems with efficacy and safety, the administration of these products to women of childbearing age and administration to healthy pregnant women is high risk and not justified.
Note: Comments placed in [ ] are added by Truth11.com editor. For example; [Flu]

Comments ()